About CXL
(formerly LS301)
A novel fluorescing molecule that binds to and illuminates cancer tissue.
How It Works
Integro’s optical imaging agent, Cronexitide Lanocianine (CXL) formerly LS301, consists of a small peptide and a dye that emits near-infrared light (NIR). CXL’s small peptide binds to the activated form of its target protein in cancer cells, aggregating the NIR emitting dye within diseased tissues and cancer-positive lymph nodes. The small molecular size enables CXL to reach cancer cells and its target protein quickly. With consistent, selective, and fast-acting accumulation in diseased tissues, CXL’s dosing schedule can be flexible. This flexibility allows CXL to be incorporated into patient care with minimal impact on operating room workflow.
Integro’s proprietary CancerVision® Goggles, as well as various commercially available imaging devices, can detect CXL’s cancer-specific fluorescent signal. The surgeon can see CXL’s fluorescence signal while operating using existing imaging equipment currently in the operating room or Integro’s wearable CancerVision® Goggles. The surgeon can be confident that all cancerous tumors and lymph nodes have been successfully removed when they no longer see any illuminated tissue.
This video showcases the intraoperative imaging of Integro's tumor-targeting CXL technology during a lung cancer surgery. The patient received CXL the day before the procedure, enabling the surgeon to use a near-infrared endoscope to detect and precisely remove the tumor.
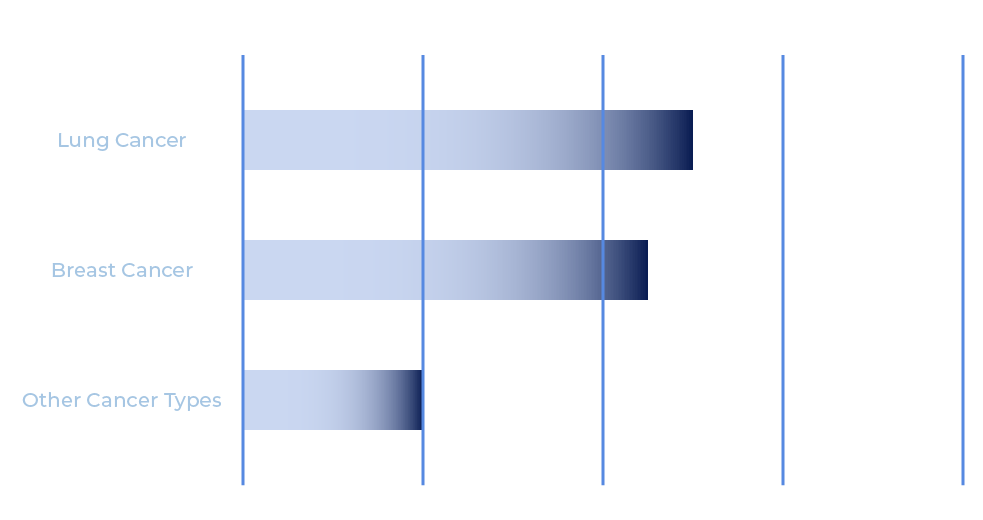
CXL as visible on various commercially available imaging devices
Designed to Identify Cancer
CXL is a stable molecule that directly targets cancer. It selectively binds to an activated protein that is uniquely expressed by cancer cells and is not found in the same form in healthy tissues. Paired with NIR light, which can penetrate human tissue, CXL illuminates cancer cells with high specificity. CXL offers significant opportunity for improved cancer imaging and more successful surgical intervention.
Enhanced Detection
The target protein transforms to its activated state when cancer cells are multiplying and in the process of spreading to healthy tissues. CXL provides the potential to locate tumors, metastases, or cancer-positive lymph nodes that would otherwise have gone undetected. Due to its high sensitivity and specificity, CXL enables more accurate identification of cancerous tissue during surgery.
Broad Indications
Unlike on normal cells, the activated protein that CXL targets is found on the surface of cancer cells. It is a critical and unique protein in cancer cells making it an ideal target for detecting diverse types of solid tumors and some blood cancers. Real-time imaging of CXL facilitates improved tumor margin assessment, surgical cavity assessment for residual tumor tissue, and visualization of sentinel and secondary cancer-positive lymph nodes.
Data Insights
Pairing CXL with imaging devices, such as Integro’s CancerVision® Goggles, offers the opportunity to store and aggregate deidentified pre-, intra-, and post-operative imaging data. This data set offers the potential to develop algorithms for advanced clinical decision support, predicting cancer spread, and personalizing patient treatment, further improving cancer surgery outcomes.
Imaging Tools Designed for Surgeons
Integro developed the CancerVision® Goggles, through the groundbreaking work by Dr. Samuel Achilefu, to support NIR fluorescence image-guided surgery without disrupting normal surgical workflow.
This wearable imaging system allows surgeons to visualize the CXL fluorescence images overlaid onto natural color images of tissues in the surgeon’s field of view via a head-mounted, mixed reality display. Surgeons can use the CancerVision® Goggles intraoperatively to visualize cancerous tissues, including tumors and lymph nodes. Tumor resection surgery guided by CXL and the CancerVision® Goggles could allow surgeons to identify even microscopic tumors and assess surgical margins in real-time, leading to more thorough tumor removal and improved surgical outcomes.
AI Powered Solutions
Optimizing the Cancer Clinical Trials with Real Time AI Powered Solutions
Integro and OmniScience accelerate clinical trials on fluorescent imaging agent to bring unparalleled precision in cancer surgery and improve patient outcomes
Integro is transforming cancer surgery through its ongoing partnership with OmniScience, a leader in AI applications for clinical trials. In this collaboration, clinical data scientists are building AI-powered computer vision workflows that allow deep tissue analysis at gross and microscopic scales and fluorescence characterization of a multitude of tissue types. By leveraging advanced analytics and AI throughout their clinical trials, Integro is working to improve the precise detection of cancer intraoperatively, by combining Integro’s proprietary CXL fluorescent imaging agent with AI-powered insights, Integro is enabling:
Identify patterns and trends through algorithms that enhance the detection of cancer with greater accuracy during surgery
Deliver data-driven clinical decision support by unifying imaging data to optimize cancer trial insights with traceable pathways
Streamline data and analysis processes to unlock new efficiencies and simplify workflows
Enable intraoperative pathology assessment through computer vision that helps identify cancer
With Integro’s proprietary CXL fluorescence imaging agent and the support of OmniScience’s AI expertise, surgeons will be able to see, analyze, and act with confidence - leading to more successful cancer surgeries and improved outcomes.